
- Home
- Special Report
- Healthtech Special
- Covid-19 test kits are getting more innovative in India. Here's how
Covid-19 test kits are getting more innovative in India. Here's how
Companies across India are concocting solutions to ramp up Covid-19 testing. To what extent can this solve issues related to access, availability, undercounting and delays?

Divya writes about gender, philanthropy, startup and workplace trends, and business from the lens of its impact on people. She is keen to find interesting stories and new ways of telling them. A journalism graduate from Mumbai who was previously with The Economic Times, Divya is also an editor and proof-reader. Outside of work, she likes to travel, read books, drink hot chocolate, and endlessly watch, read and talk about cinema.
- For businesses, sustainability should be a leadership challenge, not a cost problem: Rajeev Peshawaria
- Philanthropy for mental health should focus more on community-led models
- I was never taken seriously as someone who can build a business: Masoom Minawala
- Indian companies should focus on high value manufacturing: Vijay Govindarajan
- Roop Rekha Verma's unwavering fight to uphold constitutional values
 Hasmukh Rawal, promoter and MD of Pune-based Mylab Discovery Solutions that launched India’s first self-use Covid home test kit
Hasmukh Rawal, promoter and MD of Pune-based Mylab Discovery Solutions that launched India’s first self-use Covid home test kit
Image: Anirudha Karmakar for Forbes India
Hasmukh Rawal believes the only way to prevent a third Covid-19 wave in India is to test, isolate and vaccinate.
The promoter and managing director of Pune-based Mylab Discovery Solutions launched India’s first self-use home test kit for Covid-19 in late-May, after working on it for almost six months. “It was the first time in the history of diagnostics in India that a mobile-based AI was being used in screening of an infectious disease,” he says, explaining that normally, self-tests are conducted for blood glucose or pregnancy.
Related stories
The Rapid Antigen Test (RAT), called CoviSelf, requires users to register themselves on a mobile app. Its kit lets people take a nasal swab sample, put it in a prefilled extraction tube, add two drops of the swab sample to the test platform, and wait for 15 minutes. If the platform shows one line, the result is negative, and if it shows two, it’s positive. Results after 20 minutes cannot be considered valid. The test card also has a QR code that can be scanned on the app.
Rawal says he wants to help people test, isolate and get treated faster. “During the second wave, testing didn’t happen at the right pace, which led to many complications,” he says, adding that while the standard RT-PCR tests have to be handled by trained technicians in a laboratory, home self-test kits can “reach even villages and rural areas, where there might not be a diagnostic centre, but people have phones”.
In June, Vapi-based global medical device and health care company Meril got an approval from the Indian Council of Medical Research (ICMR) for its indigenous rapid-antigen self-test called CoviFind. The method of collecting samples and testing them is similar to Mylab’s CoviSelf, as is the time taken for test results. Data generated through these tests are stored with ICMR.
 Innovations in the diagnostics space are essential to meet the needs of India’s population, says Sanjeev Bhatt, senior vice president, corporate strategy, Meril. “Self-use RATs are easily scalable, and can thus reach areas that lack testing infrastructure or laboratories,” he says, adding that rapid antigen tests are also diagnostic solutions that are more convenient and affordable.
Innovations in the diagnostics space are essential to meet the needs of India’s population, says Sanjeev Bhatt, senior vice president, corporate strategy, Meril. “Self-use RATs are easily scalable, and can thus reach areas that lack testing infrastructure or laboratories,” he says, adding that rapid antigen tests are also diagnostic solutions that are more convenient and affordable.
Bhatt intends to make 30 to 50 lakh CoviFind tests available in the market within the first month of launch. Meril has a tie-up with Piramal Pharma, as part of which 1,200 people on-ground will educate doctors and pharmacists about the test so they can guide consumers. This is apart from distribution through e-pharmacies, ecommerce platforms and a dedicated website.
Rawal says Mylab has the capacity to make 70 lakh CoviSelf kits per week and has rolled out close to 30 lakh kits in the market. Apart from multi-linguistic information and videos to cater to people across languages and socio-economic groups, the app allows “one phone number to create an unlimited number of profiles for testing, so people who do not have a phone can access the test with the help of someone in their vicinity who does”, he says.
According to the ICMR, real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Rapid Antigen Tests (RATs) are the mainstay for Covid-19 diagnosis in India. While RT-PCR is considered the gold standard, it takes up to 24 hours in India, and requires a laboratory with skilled technicians. RATs take between 15 and 30 minutes, but are less reliable since they are not as sensitive as RT-PCR, which can detect even miniscule amounts of the coronavirus.
On May 4, at the peak of the second wave, the ICMR had stated in an advisory that, “At present, laboratories are facing challenges to meet the expected testing target due to extraordinary case load and staff getting infected with Covid-19. In view of this situation, it is imperative to optimise the RT-PCR testing and simultaneously increase the access and availability of testing to all citizens of the country.” It also suggested that in this scenario, RATs, with their short turnaround time, have the advantage of quick detection of cases and opportunity to isolate and treat early for curbing transmission.
Then on July 5, the ICMR said that about 120 RAT kits had been validated and found to be satisfactory. Dr Viswesvaran Balasubramanian, consultant for interventional pulmonology and sleep medicine at Yashoda Hospitals Hyderabad, says RATs have a higher chance of missing a positive Covid-19 case, especially in those with low viral load. “Though still a matter of research, few manufacturing companies have reassured that the accuracy of diagnostic tests used to detect Covid-19 was unlikely to be affected by fast-spreading mutant strains of the virus,” he says.
Companies offering different RT-PCR or RATs claim they save cost, time and manpower, while enabling scaling up and improving access. Some examples include Tapestry Pooling by Bengaluru-based Algorithmic Biologics, which collects less-invasive, self-swabbed shallow nasal samples from multiple individuals to be pooled and tested in a single round using RT-PCR. There is the PanBio RAT device by Abbott, approved by ICMR, for home tests for Covid-19. Then there is Thermo Fisher Scientific, which launched a rapid point-of-care testing platform in June that uses the RT-PCR method to provide visual results in 30 minutes.
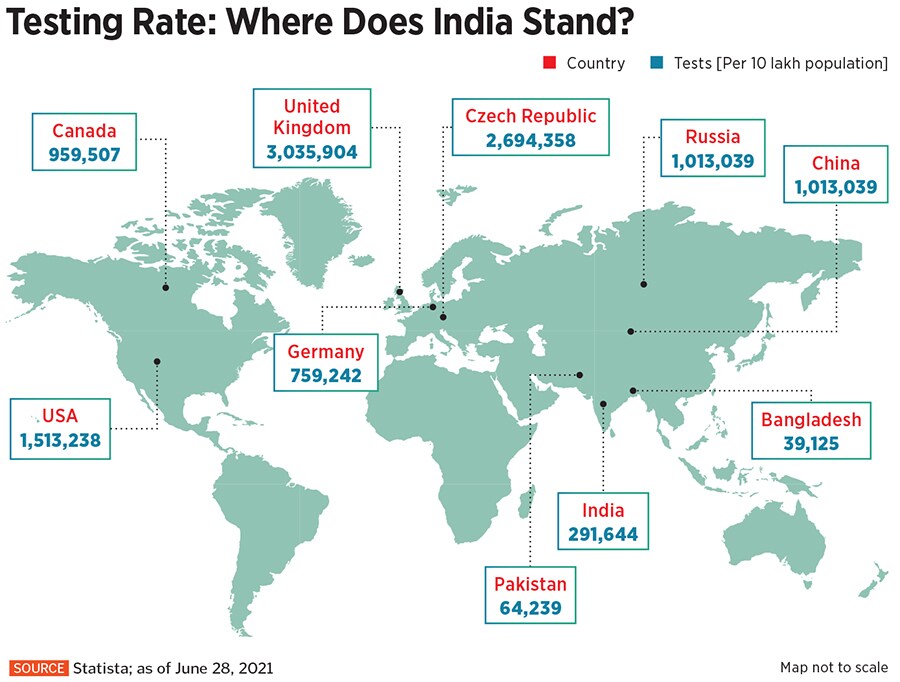
Can They Reduce Testing Woes?
India’s testing capabilities have increased manifold since the beginning of the pandemic in March 2020. Daily testing numbers have exceeded 19 lakh, with a total of more than 40 crore since March last year, says Dr Balasubramanian. He adds, however, that the number of people tested per million (10 lakh) population remains low.
Last September, for instance, when the single-day count of Covid-19 cases reached 98,000, ICMR data says India was conducting 11 lakh tests per day. The number of daily cases increased by over four times since, reaching a high of 3.8 lakh in April, while India’s testing capability did not even double.
“Another point of concern is that the number of positive cases per 100 samples tested is around 7.35 percent, well above the recommended level of 5 percent,” says Dr Balasubramanian, explaining that one reason for this could be because only symptomatic people are getting tested, while asymptomatic ones continue to spread the infection. “A multitude of issues related to capacity, data monitoring, skilled human resource and supply chain constraints pose major challenges to strengthening testing capacity.”
India’s testing infrastructure was not equipped to fight sudden major surges in cases, and the country’s testing strategy has been more reactive than proactive, says Ameera Shah, promoter and managing director of diagnostics company Metropolis Healthcare. “It takes around 45 days to two months to build or upgrade a Covid lab for new capacity. Therefore, it is difficult for any lab, even of scale, to expand their capacity overnight. By the time the capacity is expanded, the surge is already over,” she explains. “The planning must be done much in advance.”
 Manoj Gopalkrishnan, founder of Algorithmic Biologics
Manoj Gopalkrishnan, founder of Algorithmic Biologics
Shah believes the government must have more frequent dialogues with stakeholders to take stock of the on-ground situation. She says in the wake of the second wave, she saw doctors confused about how to diagnose patients, given the evolving nature of the virus; there was pressure from consumers who wanted reports as soon as possible, while broken supply chains meant testing equipment and chemicals were not reaching labs on time, and health care workers were getting infected. With laboratories getting overwhelmed, people are spending more time waiting for results.
“In terms of testing, time is a variable that has not been fully appreciated,” believes Manoj Gopalkrishnan, founder of Algorithmic Biologics, who has developed Tapestry to pool test samples in a bid to speed up and scale up coronavirus testing, while reducing costs. “Because if one person is Covid-positive and you give that person 12 more hours to spread that infection in the community [due to delays in testing], you might end up with eight more cases on your hands.”
Suppose one person in a group of 16 has contracted Covid-19. Individually, this would mean conducting 16 different tests. Tapestry Pooling attempts to identify who is positive in just one-fifth the number of tests. “We combine samples in different ways so that every sample ends up going to different cohorts,” explains Gopalkrishnan, a professor at IIT-Bombay. “For example, you could arrange these 16 samples in a grid of 4*4, test all four samples in each row together and then each column. So you’ve done eight tests in total. Exactly one row will be positive, corresponding to the row of the person who tests positive, and exactly one column will be positive. From this information, you have uniquely picked out that one person who is positive.”
Gopalkrishnan is focusing on educational institutions, commercial office spaces and apartment complexes for deployment of Tapestry Pooling. “It has merit in any place where people see value in gradual reopening and resuming social interactions. Especially in a post-vaccination world, where we will look at those rare individuals who have the infection in an attempt to detect some early variant of the virus, this kind of testing becomes an important strategy to complement vaccination.”
The solution involves weekly re-testing of individuals. “In that kind of situation, we do not expect more than 10 people to test positive. So if there are 1,000 people on campus and 10 or fewer have Covid-19, that’s where pooling really shines,” he says, adding that the biggest advantage is perhaps how the method allows a lab with a certain capacity to massively increase the number of samples it can test without buying new material or equipment, or hiring more people.
The idea is to reduce the time taken to test pooling samples using RT-PCR to 12 hours from the current 24 hours. “We also believe we can bring down the cost of testing by half in campus screening use cases. The goal is that if people on a campus are getting tested every week, then in a month each person should not be paying more than ₹1,000,” says Gopalkrishnan.
 Thermo Fisher’s reusable Accula Dock streamlines RT-PCR testing using a fully integrated, single-use microfluidic test cassette
Thermo Fisher’s reusable Accula Dock streamlines RT-PCR testing using a fully integrated, single-use microfluidic test cassette
Dr Balasubramanian says pooling samples of individual PCR specimens from high-risk groups such as migrant workers and international passengers in institutional quarantine facilities—followed by individual specimen testing only if a pooled sample tests positive—can save time and resources, and help understand disease patterns. That said, he believes lack of skilled lab personnel and delayed reporting remain major hurdles. “Adhering to the recommendations of ICMR and usage of standardised kits might mitigate the problem to some extent.”
Testing requirements are evolving in various settings as per changing travel regulations, lockdown norms, health care needs, and with the emergence of variants, a standard lab-based RT-PCR test will need to be complemented with rapid point-of-care tests which are as accurate, says Amit Chopra, managing director, India and South Asia, Thermo Fisher Scientific.The company’s rapid point-of-care RT-PCR testing platform—Accula system, was launched in India in June. It has been developed by the US-based Mesa Biotech, which was acquired by Thermo Fisher earlier this year.
The testing platform has received an emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for the detection of the coronavirus in Clinical Laboratory Improvement Amendments (CLIA)-waived environments. The reusable Accula Dock, which can also be powered by a portable battery, is imported from the US, and streamlines RT-PCR testing using a fully integrated, single-use microfluidic test cassette. The final pricing will be determined by entities undertaking testing. The test uses nasal swab samples to provide visual results in 30 minutes.
It can be used in places like airports, ICU settings, ambulances, defence and sports arenas, amongst numerous other applications, Chopra explains. “For example, the rapid test is being used by the Cochin International Airport in Kerala, and will be very helpful as some countries may implement a protocol that mandates a rapid PCR negative certificate taken within four hours before departure for passengers travelling from India. This is not possible with standard RT-PCR equipment,” he says. Another example of the point-of-care platform is when patients are admitted to emergency care units in hospitals, and doctors need immediate test results to determine whether the patient needs a Covid ICU or non-Covid ICU. “The National Football League in the US is using this technology to screen players, spectators etc. Rapid testing is required in environments and scenarios where you cannot carry bulky machines or labs, but you need equally accurate results very fast.”
 A Fine Balance
A Fine Balance
A lot of new tests and technologies have been launched due to the urgency of the Covid-19 crisis, but not maintaining the highest standards could result in many active cases being falsely reported as negative, says Shah of Metropolis Healthcare. “For example, the glucometer has existed for a long time, but diabetes patients still often prefer to come to a lab and confirm whether their blood sugar levels are correct, as the glucometer does not always have the same level of accuracy as a lab test,” she says, adding that Covid home self-care tests have been developed in a much shorter time period. “There will be a reason and need for both [PCR and home self-test kits] in different contexts and settings, but PCR will always remain the gold standard.”
The ICMR has set a minimum acceptance criteria for sensitivity and specificity of rapid antigen tests. Sensitivity is a test’s ability to correctly generate a positive result for people who have the condition they are being tested for, while specificity is a test’s ability to correctly generate a negative result for people who don’t have the condition they are being tested for. For point-of-care tests that do not require transport to a laboratory, the ICMR has a 50 percent and above threshold for sensitivity and 95 percent and above for specificity.
The ICMR guidelines also suggest no repeat testing is required if RAT results are positive. But if a person is symptomatic and the RAT result is negative, then an RT-PCR is recommended. Dr Balsubramanian says, “Such sequential testing with an RT-PCR after a negative RAT report might increase the expenditure for screening.”
Going forward, prioritising last-mile access to diagnostic offerings will be an important task for the industry, stresses Bhatt of Meril. Another area with a potential for growth, according to him, is strengthening the diagnostics’ value chain to enable a “smooth, streamlined, robust and well-connected network that supports the delivery of medical diagnostics and devices”.







