
How Raman Effect Can Help Detect Bombs
In two separate studies, Indian scientists have used Raman spectroscopy to detect hidden substances and to aid drug discovery
Around nine decades ago, a young man called Chandrasekhara Venkata Raman from the town of Tiruchirappalli in Tamil Nadu was sailing back to India after attending a conference in London. Raman was already a physicist of repute at the University of Calcutta. As the story goes, during the voyage, Raman would often sit on the ship’s deck and gaze at the azure of the Mediterranean Sea. Eventually, and perhaps inevitably, given his interest in the study of light, Raman began to wonder where the sea got its colour from.
The prevailing notion at the time was that the blue of the sea was a reflection of the sky. As for the sky’s colour, physicist Lord Rayleigh had won a Nobel Prize in 1904 for proposing that it was due to minute particles in the air scattering the blue wavelength from the sun’s white rays, while absorbing all other wavelengths of colour. But Raman had a hunch that the sea’s hue was more than just a reflection of the sky. Using a Nicol prism, he eliminated some of the light from the sky that the sea reflected. This made the water’s colour grow even more intense. “The hue of the water is of such fullness and saturation that the bluest sky in comparison with it seems a dull grey,” Raman wrote in an article in the journal Nature in 1921, describing his observations with the Nicol prism. Clearly, water was responsible for its own colour, rather than merely reflecting the sky’s.
Over the next seven years, Raman and his students—including physicist KS Krishnan—shone light onto several transparent liquids, ranging from water to glycerine. Each time, they saw a faint glow: Blue in the case of water, green in the case of glycerine and so forth. Finally, on February 28, 1928, celebrated as Indian Science Day, Raman described to a group of scientists in Bangalore the phenomenon that would win him India’s first Nobel for science. Although Krishnan contributed greatly to the discovery as a co-discoverer of the Raman Effect—and for which he is widely acknowledged—he didn’t share the Nobel.
Raman Scattering or Raman Effect—as the phenomenon was eventually named—is the scattering of light that falls on a substance, leading to a change in the colour of the light that emerges from the substance. Normally, a large part of incident light gets absorbed, transmitted or reflected. But a tiny part—about one out of every 10,000 photons (light particles) that fall on a substance—undergoes Rayleigh scattering. This means the photon gets scattered, but doesn’t change its wavelength and, therefore, its colour. An even tinier part—about one photon in 10 million—not only scatters, but also changes its wavelength and colour. What’s more, the change in the colour of the light is decided by the substance that scatters the light. When the substance is water, for example, the light turns blue. By studying the altered light, therefore, one can identify the substance as water.
Raman spectroscopy, which uses this phenomenon to detect various substances, is today used in everything from quality control in the pharmaceutical industry (examining active pharmaceutical ingredients in drugs) to medical diagnostics like understanding the composition of tumours in cancer patients. Physicists continue to tweak this powerful technique while being on the constant look out for new applications, and new ways of observing the Raman Effect closely. Hundreds of papers are written each year, several of them by Indian scientists.
Joining the ranks this year are two groups of scientists, one from the Indian Institute of Science (IISc) in Bangalore, and the other from the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) in Bangalore and the University of Mysore. While IISc researchers showed how Raman spectroscopy can identify dangerous substances such as improvised explosive devices (IEDs) at airports and border checks, scientists from JNCASR and the University of Mysore showed how it can help drug discovery. Both methods shine new light on the problems of imaging opaque substances and screening compounds that hold the potential to form new drugs.
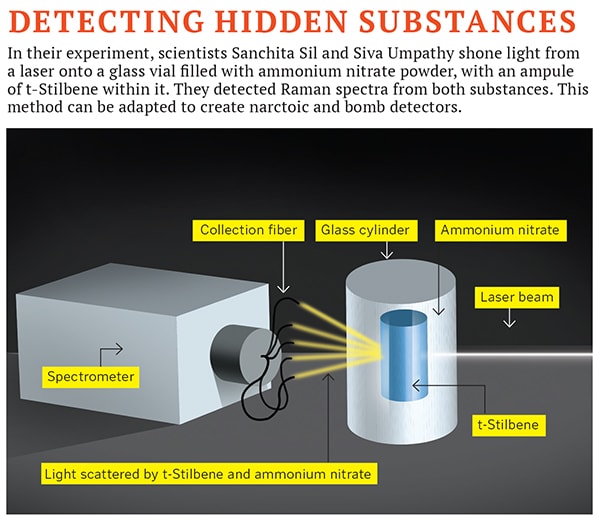
What lies Beneath
Raman spectroscopy had not been used earlier to identify substances with multiple layers, such as a tumour tissue hidden under a layer of skin, or a second layer of paint hidden beneath the visible surface of a painting. This is because when Raman spectroscopists train a laser on a substance, they only analyse the light scattered back in the direction of the laser. As a result, only the surface of the substance is analysed. “If there is something of interest hidden below, for example, explosives inside a polymer container, a conventional Raman instrument will not detect it,” says Freek Ariese, a Raman spectroscopist at VU University, Amsterdam.
A couple of months ago, though, scientists at IISc found a solution. In the June issue of Scientific Reports, spectroscopists Sanchita Sil and Siva Umapathy published a paper, describing a new arrangement of light-collection fibers to study Raman spectra after light penetrates a substance. They concealed an ampule of the organic compound t-Stilbene inside a glass cylinder filled with ammonium nitrate, an explosive often used in IEDs. Older Raman spectroscopy methods wouldn’t have detected the t-Stilbene ampule through the explosive. But Sil and Umapathy did a few things differently. After shining a laser onto the cylinder, they collected the emitted spectra with light-collection fibers placed in a 3600 plane around the cylinder, instead of observing only that light which is scattered back towards the laser. The Raman spectra of t-Stilbene was strong enough to be detected through the ammonium nitrate.
This showed how Raman spectroscopy can be used to image objects with multiple layers. If t-Stilbene showed up through half a centimetre of ammonium nitrate, other hidden substances would too.
This method could have many applications. Fliers, for instance, can’t carry bottles of liquids because there is no way of knowing if the liquid is an explosive or a narcotic. X-rays only reveal the density of the contents, but cannot distinguish between two substances of similar densities. “If you have half a kilogram of halwa in your bag, it might look like RDX on an X-ray scanner,” says Umapathy. This is why, when an object has a suspicious X-ray profile, the only way baggage screeners can confirm what is inside the bag is by opening it up.
This wouldn’t happen if the detection process could tell the chemical composition of the substance, like Umapathy’s method does. UMARS or Universal Multiple Access Raman Spectroscopy, as the method is called, will make it possible to identify the composition of liquids inside bottles, making water-bottles and shampoo containers kosher on flights again. UMARS can also be adapted into narcotic detectors. “In border areas, migrants often carry packages while crossing over. Security personnel don’t have the time to open all bags. One of the things you can have is a handheld instrument that can scan the luggage without opening all of it. These technologies can be developed,” says Umapathy. He explains that security personnel could develop a database of the Raman spectra of various contraband substances (each forbidden substance would have a unique spectra) and use the database to identify suspicious substances.
The researchers have applied for patents for these ideas.
Sil and Umapathy have some way to go before their method is ready for the field, say other researchers. Sudipta Maiti, an expert in imaging technologies at Mumbai’s Tata Institute of Fundamental Research, says that while Sil’s and Umapathy’s research is interesting, t-Stilbene, the compound used in their experiment, is a “good Raman emitter”; because it is a dye, t-Stilbene’s Raman spectra is strong and easily detectable. The real test for UMARS would be if the experiment was reversed, and ammonium nitrate was placed in the inner ampule; ammonium nitrate has a weaker Raman spectra and would be more difficult to detect. “Several other substances you may want to detect may or may not be good Raman emitters,” Maiti says. “In that case, it becomes a problem for this method. More work needs to be done by the researchers to overcome this.”
Umapathy and Sil, though, believe they are close to creating field-ready applications. They are already in talks with the industry for partnerships to develop the idea.
A Closer Look at Drugs
In February, a team of scientists from Bangalore’s JNCASR and the University of Mysore found another use for Raman spectroscopy in drug discovery. In their paper published in the Proceedings of the National Academy of Sciences, the researchers showed the way in which a well-known drug for hypertension called Felodipine was working against cancer, paving the way for new anti-cancer drugs based on the same mechanism.
Felodipine has been used by doctors since the 1980s to control high blood pressure. In 2005, a study on Chinese patients found that Felodipine users had a 36 percent lower chance of developing cancer. Despite its potential, Felodipine hasn’t been used as an anti-cancer drug yet, “for the simple reason that it is a hypertension drug; so a person who does not have hypertension may then develop low blood pressure,” says Chandrabhas Narayana, one of the authors of the paper and a professor at JNCASR. “To avoid this side effect, pharmaceutical researchers need to understand exactly how Felodipine works against cancer, so they can design a new drug on that principle.”
Narayana’s group knew that a protein called Aurora A kinase, found in large quantities in cancer cells, could be helping the cancer grow. They wanted to see if Felodipine was fighting cancer by binding to this protein and paralysing it. This is where they turned to Raman spectroscopy. They studied the Raman spectra of Aurora A kinase molecules before and after Felodipine bound itself to it, and saw different spectra in both cases. The spectra are a rich source of information. By studying it, the researchers could also deduce where on the body of the Aurora A kinase molecule Felodipine was attaching itself.
Next, they used software that simulates the binding of Felodipine to Aurora A kinase, and found that it confirmed their deductions: That Felodipine indeed attached itself to, and paralysed, Aurora A kinase molecules; and where on the protein molecule it latched on to.
“This research is still in a nascent stage,” says Narayana. “People will have to develop a good understanding of our method, but then it can become a very nice tool for pharma companies.” In their quest for new drugs, researchers analyse thousands of molecules to figure out if they have an effect on a disease. By using Narayana and his group’s method, researchers can greatly reduce the time spent on the screening process.
The advantage of Raman spectroscopy, which attracts researchers like Narayana, Sil and Umapathy, is that it is both fast and non-invasive. “This makes it a preferred technique when time is limited [such as a 24-hour process control in a factory with continuous feedback] or when sample damage should be avoided [art authentication and gem identification],”explains Ariese. Such ease of use is why, nearly a century after Raman wondered about the colour of the sea, his discovery continues to be the fount of new applications.
(This story appears in the 30 November, -0001 issue of Forbes India. To visit our Archives, click here.)





