
Covid-19 ventilators, made in—and for—India
Noccarc Robotics and Biodesign Innovation Labs have built affordable ventilators from scratch to meet Indian needs, while meeting global standards
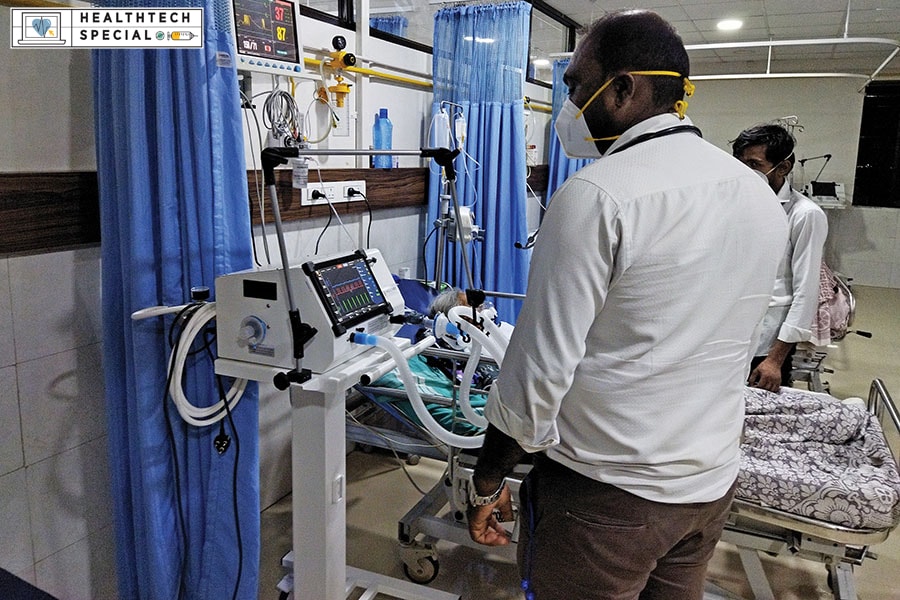 Noccarc Robotics’s V310 ventilator deployed at a remotely located hospital in Bijapur, Karnataka
Noccarc Robotics’s V310 ventilator deployed at a remotely located hospital in Bijapur, Karnataka
When the Covid-19 pandemic arrived on Indian shores in 2020, the country was caught off-guard on several fronts. One of them was the acute shortage of ventilators. In April 2020, the Center for Disease Dynamics, Economics & Policy (CDDEP), which produces independent and multidisciplinary research, estimated India had about 1.9 million hospital beds, 95,000 ICU beds, and 48,000 ventilators.
With Covid-19 still an unknown element across the world, and experts uncertain of ways to tackle it, ventilators emerged as an immediate way of treating critical patients. Expecting a massive surge in demand for ventilators in the coming months, the government of India, in March 2020, called on Indian companies to rapidly scale up manufacturing capacities and fill the supply gap. India Inc rose to the challenge, with established ventilator manufacturers as well as startups ramping up production capacity, inventing entirely new machines, and collaborating with other industry players in unprecedented ways.
Conventionally, ventilators are expensive and highly sophisticated machines, with high-end tertiary hospitals in big cities usually using imported variants that cost between ₹10 lakh and ₹30 lakh. Once installed, these complex machines cannot be moved around within the hospital, depending on changing requirements, and need trained technicians to operate and monitor them. These factors make it difficult for smaller hospitals, and those in smaller cities and towns, to afford and operate these machines.
The Covid-19 crisis presented an opportunity to fledgling Indian startups to take up the challenge of technological innovation and create ventilators that are at par with any world-class product, but better suited to Indian needs and conditions, and available at a fraction of the cost of imported variants. Their aim has been to create ‘made-in-India’ products of superior quality that meet global standards and certifications, while keeping them affordable for hospitals of all sizes and capabilities.
(This story appears in the 30 November, -0001 issue of Forbes India. To visit our Archives, click here.)




 IIT- Kanpur graduates Harshit Rathore (left) and Nikhil Kurele founded Noccarc Robotics in 2017
IIT- Kanpur graduates Harshit Rathore (left) and Nikhil Kurele founded Noccarc Robotics in 2017 Kurele says while government specifications and certifications ensure that an equipment meets the requirements, they do not specify the quality of the product; it is up to the manufacturers to produce equipment that is of superior quality. “What we are trying to do is comply with US and EU certification standards, which will automatically raise the standard of the product,” he says. “Most medical equipment in India are imported, and hence are designed for European and US markets. Our designs keep in mind the ground requirements of India, with the quality standards of global markets.”
Kurele says while government specifications and certifications ensure that an equipment meets the requirements, they do not specify the quality of the product; it is up to the manufacturers to produce equipment that is of superior quality. “What we are trying to do is comply with US and EU certification standards, which will automatically raise the standard of the product,” he says. “Most medical equipment in India are imported, and hence are designed for European and US markets. Our designs keep in mind the ground requirements of India, with the quality standards of global markets.”



